COVID-19 Standard, Alpha & Beta Variants Test Kit
Product Cat. No.: RP-011. | CE-IVD Registration No.: RPS/499/2021.
Novel Coronavirus 2019-nCOV Nucleic Acid Typing Test Kit
(For Covid-19 Standard, UK & South Africa Variants Detection)

[Product Name] Novel Coronavirus 2019-nCOV Nucleic Acid Typing Test Kit (Real-Time PCR Method).
[Packing Specifications] 100 Tests/Box.
[Intended Usage]
This kit is used for the in vitro qualitative detection of suspected cases of pneumonia caused by New Coronavirus infection, patients with suspected clusters, other patients who need to be diagnosed or differentially diagnosed with the New Coronavirus infection, and nasopharyngeal swabs, oropharyngeal swabs, and bronchoalveolar lavage fluid samples of patients with mutation beads for the New Coronavirus ORF1ab and N gene, S gene N501Y mutation and S gene E484K mutation sites.
[Detection Principle]
This kit is designed with two pairs of specific primers and Taqman probes for the New Coronavirus ORF1ab and the conservative region of the N gene sequence encoding the nucleocapsid protein of the new coronavirus to diagnose or differentially diagnose whether the tested patient is infected with the new coronavirus, the British B.1.1.7 strain-specific N501Y mutation site and the South African B.1.351 strain-specific E484K mutation sites.
The PCR reaction system contains primers and probes for internal standard. The process of sample collection, extraction and reaction is monitored by detecting internal standard to avoid false negative results.
[Product Main Components]
Kit composition
|
Component name |
Specifications |
Quantity |
Main components |
|
Reaction solution |
875μL |
2 Tubes |
Specific primers, probes, 2 x RT PCR Buffer |
|
Enzyme mix solution |
125μL |
2 Tubes |
Reverse transcriptase, DNA polymerase |
|
Negative control |
400μL |
1 Tube |
Pseudovirus containing internal control fragment |
|
Positive control |
400μL |
1 Tube |
Pseudovirus containing target fragment and control standard fragment |
|
Instructions Manual |
- |
1 copy |
- |
Required but not provided: Nucleic acid extraction or purification reagents.
Note: 1. The components of different batch kits cannot be used interchangeably.
2. The positive control and the negative control need to be used in the nucleic acid extraction.
[Storage Conditions & Validity]
1. The kit should be stored frozen at -20℃±5℃ and protected from light; the expiration date is 6 months; the production date and expiration date are shown in the outer packaging box.
2. Avoid repeated freezing and thawing of the kit and the number of freezing and thawing shall not exceed 7 times.
3. After opening, the bottles should be stored at -20℃±5℃ and protected from light. The number of bottles opening times should not exceed 7 times, which will not affect the use within the validity period.
[Applicable Instruments]
1. This kit has been validated on ABI7500 quantitative fluorescence PCR instrument.
2. For other models not listed, relevant experiments have not been performed or completed for this kit. If users need to use this type of instrument platform to carry out the detection of this reagent, please contact our Technical Department at cs@healthcare-bio.com for relevant support.
NB: The other devices can include quantitative fluorescence PCR platforms with FAM, VIC, ROX and Cy5 channels.
[Sample Requirements]
1. Nasopharyngeal swabs, oropharyngeal swabs and other methods are used to obtain samples, and it is recommended to use commercial virus sampling kits for sample collection devices. The specific operation method is as follows:
i) Nasopharyngeal swabs: Nasopharyngeal swab: The operator gently rotates the sterile swab to the left and right at the angle parallel to the upper jaw and inserts it from one nostril to the nasal palate in the nasal passage. Generally, the swab stays when there is resistance to the insertion Rotate slowly to exit after 2-3s.
ii) Oropharyngeal swab: The operator holds the tongue depressor and presses the back root of the patient's tongue in one hand, and holds the root of the sterile swab in the other hand, and then quickly and firmly apply the sterile swab to the left and right sides and the swab head. Scrape the secretions on both sides of the tonsils and the back wall of the pharynx.
iii) Place the nasopharyngeal swab or oropharyngeal swab into the centrifuge tube containing the preservation solution for later use.
2. Alveolar lavage fluid sample: Use a sterile syringe to extract the sample and place it in a centrifuge tube for examination.
3. Cross-contamination between samples should be avoided.
4. Samples should be tested in time after collection, or stored at -20±5℃ for testing, and kept below -70℃ for long-term storage.
[Testing Method]
1. Reagent preparation: (Reagent preparation area)
In each PCR reaction, both the positive control and the negative control are tested simultaneously, and each sample needs to be tested for the ORF1ab region, N gene, S gene N501Y mutation, S gene E484K mutation and internal standard.
1) Remove the kit from the refrigerator, equilibrate at room temperature, fully dethaw the components, vortex to mix and then centrifuge instantly.
2) Calculate the number of reactions required for the current experiment n (n = number of samples + negative control + positive control), and mix the reagents according to the preparation method of the reaction system in Table 1 and then divide into 20μL/well PCR reaction tube. Transfer the PCR reaction tube to the sample preparation area, and put the remaining reagents back to -20℃±5℃ and keep away from light.
Table 1: Reaction system preparation method
|
Composition |
Volume |
|
Reaction solution |
17.5μL |
|
Mixed enzyme solution |
2.5μL |
|
Total volume |
20μL |
2. Sample preparation: (Sample preparation area)
1) RNA extraction:
· It is recommended to use commercially available nucleic acid extraction kits to extract RNA sample for PCR detection. Extract according to the operation steps of the Extraction Kit instructions.
· The positive control and negative control of this kit are included in the extraction.
2) Loading/Sample addition:
a. Take out the reagent prepared in the reagent preparation area and centrifuge at low speed for 10 seconds.
b. The RNA of the sample to be tested, the positive control and the negative control after treatment are respectively added into the PCR reaction tubes at the volume of 5μL/well.
c. Cover the PCR reaction tubes, record the sequence of template loading, and centrifuge at low speed for 10 seconds.
d. Transfer the PCR reaction tubes to the nucleic acid amplification area for operation.
Note: Avoid contamination during RNA sample extraction and loading. If the extracted RNA template cannot be immediately tested, it is recommended to store at or below -70oC.
3. PCR: (Nucleic acid amplification area)
1) Boot, preheat and check the performance of the instrument.
2) Take the PCR reaction tubes prepared in the sample preparation area and place them in the corresponding positions in the sample tank of the machine (Pay attention to check whether the reaction tubes are tightly closed before loading and starting the machine to avoid aerosol pollution of the instrument and the environment due to PCR products leakage) and record the placement order.
3) Set the nucleic acid amplification relevant parameters in the machine according to Table 2 and carry out PCR amplification.
Table 2: Instrument nucleic acid amplification related parameters
|
System |
Set the reaction system to 25μL |
||
|
Signal acquisition |
- Select FAM channel to detect the ORF1ab and N gene of the virus. - Select the VIC channel to detect the E484K mutation of the virus S gene. - Select ROX channel to detect the N501Y mutation of the virus S gene. - Select Cy5 channel to detect internal control. |
||
|
PCR reaction conditions |
Stage |
Conditions |
Number of cycles |
|
UDG reaction |
25℃: 5min |
1 |
|
|
Reverse Transcription |
50℃: 5min |
1 |
|
|
Pre-denaturation |
95℃: 3min |
1 |
|
|
PCR |
95℃: 5s |
45 |
|
|
60℃: 34s (Collect fluorescence signal at the end of this step) |
|||
Note: ABI series fluorescence quantitative PCR instrument does not choose ROX calibration, and select <None> for quenching group.
4. Reading of test results
For data analysis, select ΔRn VS Cycle and Linear mode; select Auto Threshold and Auto Baseline, view the fluorescence curve, and obtain the fluorescence quantitative curve and Ct value in the fluorescence quantitative PCR instrument.
5. Quality control procedures
1.
Positive control: FAM, VIC and ROX signals have obvious amplification curves,
Ct value ≤32, Cy5 signal has or does not have an amplification curve.
2. Negative control FAM, VIC and ROX signals have no rising curve; Cy5 signal
has a significant amplification curve, Ct value ≤32.
3. The above requirements must be met at the same time in the same experiment,
otherwise, the experiment is invalid and needs to be carried out again.
6. Interpretation of results
1. According to the below table, determine the detection results of the new coronavirus.
|
Test results |
Test results and interpretation |
|||
|
ORF1ab
and N genes |
S gene N501Y ( ROX channel) |
S
gene E484K |
Internal Control (Cy5 channel) |
|
|
- |
- |
- |
+ |
Negative |
|
+ |
- |
- |
+ |
Positive, but not B.1.1.7 and B.1.351 mutant strains (if FAM channel 40 ≥ Ct value >36, repeat testing is required) |
|
+ |
+ |
- |
+ |
Positive B.1.1.7 mutant strain |
|
- |
+ |
- |
+ |
Suspected B.1.1.7 mutant strain, repeat the test |
|
+ |
+ |
+ |
+ |
Positive B.1.351 mutant |
|
- |
+ |
+ |
+ |
Suspected B.1.351 mutant strain, repeat the test |
|
- |
- |
- |
- |
Invalid |
(+): Presence of amplification S shape curve; (-): Absence of amplification S shape curve
2. The sample with the FAM channel 40 ≥ Ct value >36 needs to be sampled and tested repeatedly. If the retest result is consistent with the previous result, the sample test result is Positive, but not for B.1.1.7 and B.1.351 mutant strains. If the retest result is negative, the sample test result is Negative. If the retest results FAM, VIC, and ROX channels have all amplification curves, and the Ct value is ≤40, the sample test result is Positive for B.1.351 mutant strain. If the retest results of FAM and ROX channels have amplification curves, and the Ct value is ≤40, then the test result of the sample is Positive for B.1.1.7 mutant strain.
3. Samples with negative ORF1ab and N genes need to be sampled and tested repeatedly. If the retest result is positive for ORF1ab and N genes or consistent with the previous result, the sample test result is Positive. If the retest result is negative, the sample test result is Negative.
4. If the internal standard Cy5 does not have an amplification curve or Ct >40, the test result of the sample is invalid. The reason should be found and eliminated, and the experiment should be repeated for the concerned sample.
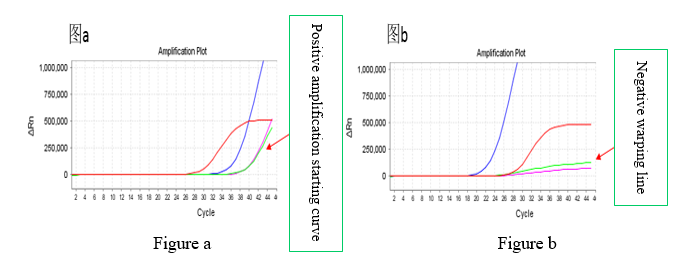
5. When interpreting the test results, it is necessary to distinguish between positive amplification starting curve and negative warping line. The channel shown in Figure a is a positive amplification curve, and the channel shown in Figure b is a negative warping line.
[Positive Value Determination]
1. Positive result: The fluorescent quantitative curve has amplification curve, Ct
value ≤40.
2. Negative result: The fluorescent quantitative curve has no amplification
curve or Ct value >40.
[Test Results Interpretation]
1.
Negative and positive controls should be tested in each experiment and the test
result can be determined only when the controls meet the quality control
requirements.
2. When FAM, VIC and ROX channels are positive, Cy5 channel result may be
negative due to the system competition.
3. When the internal standard result is negative, if the FAM, VIC and ROX
signals of the test sample are also negative, the test result of the sample is
invalid, and the cause should be found and eliminated, and the experiment
repeated for this sample.
[Product Performance Index]
1. Precision: The coefficient of variation Intra-batch / Inter-batch, Intra-day / Inter-day among different operators shall not be higher than 5.0%.
2. Coincidence rate of negative positive controls: The coincidence rate of positive controls and negative controls is 100%.
3. Minimum detection limit: The minimum detection limit of this kit is 500 copies/mL.
[Test Method Limitations]
1. The test results of this kit are for clinical reference only. The clinical diagnosis and treatment of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests, and treatment response.
2. The improper operation of the sample during the collection, transportation, storage and nucleic acid extraction process can easily cause RNA degradation and lead to false negative results.
3. When the detected nucleic acid concentration in the sample is less than the minimum detection limit, false negative results may occur.
4. If cross-contamination occurs during sample collection and preparation, it is easy to lead to false positive results.
5. Some infected people have a large number of dead virus in their samples due to the taking of antiviral drugs. At this time, there may be strong positive results detected by this kit and negative results detected by the culture method. In such case, the recent medication situation of the tested person shall be inquired.
6. Variations of the targeted sequence of the virus to be tested or other cause of sequence changes may lead to false negative results.
7. For a new type of virus, the most suitable sample type and the best sampling time after infection may not be confirmed. Therefore, the possibility of false negative results will be reduced if the samples are collected in the same patient in different times and multiple parts.
[Precautions]
1. Laboratory management shall be strictly implemented in accordance with the management specifications of the “Administrative Measures for Clinical Gene Amplification Laboratory of Medical Institutions” promulgated by the General Office of the Ministry of Health.
2. Laboratory personnel must have professional training and have certain experience.
3. The experimental process should be carried out in zones (reagent preparation zone, sample processing zone, nucleic acid amplification zone). Special instruments and equipment should be used at each stage of the experiment operation, and supplies should not be used in each stage. There should be strict requirements to minimize cross-contamination.
4. Experimental consumables (such as centrifuge tubes, suction heads, etc.) should have reasonable cleaning and quality inspection procedures to prevent contamination from causing false positive results or amplification reaction inhibitors to cause false negative results.
5. The instrument and its supporting power supply system should be checked before use to ensure the normal operation of the reagent after the reagent is out on the machine.
6. The suction heads used in the experiment shall be directly put into the waste tank containing 10% sodium hypochlorite and discarded together with other waste products.
7. The workbench and various experimental items are often disinfected with 10% sodium hypochlorite, 75% alcohol and UV lamps.
8. The Real-Time PCR machine requires frequent calibration and sample plate wells (holes) cleaning.
9. To prevent fluorescence interference, avoid direct contact with the octaplex PCR reaction tubes and tubes covered by hands.
10. The positive control in this kit is not contagious and will not cause harm to the human body. However, it is recommended to treat it as a potentially infectious substance when using it.
11. The test samples involved in this kit should be considered as infectious substances, and their handling must meet the relevant requirements of the General Guidelines for Biosafety of Microbiology and Biomedical Laboratories of the Ministry of Health and the Medical Waste Management Regulations.
[Manuscript version and approval date]: Manual version: V1.0; Date: 23 February 2021.

